In Depth
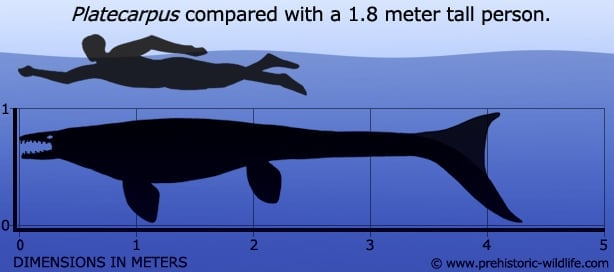
Research into Platecarpus has arguably advanced our understanding of the mosasaurs more than any other genera, part of this being down to the number of known Platecarpus fossils. Some of these specimens of Platecarpus are so well preserved that they actually reveal impression of soft tissues of body parts like the internal organs. This level of preservation was down to the relatively small size of Platecarpus (four meters may sound big but compare that to the fifteen meter plus monsters such as Tylosaurus at the larger end of the scale and you’ll have an idea) which made it easier for the body to be rapidly buried by sediment. Burying of the body is vital for the fossilisation process as without it the remains are destroyed by a combination of scavengers feeding off the body and environmental factors like erosion of the bones.
A Platecarpus fossil (LACM 128319) not only has a scleral ring (the bony structure that supports the shape of the eye) but possibly also remains of the retina. This retina also shows the presence of what are thought to be melanosomes, a cell that contains melanin, study of which may one day reveal the colour of Platecarpus’s eyes. Impressions of what appear to be internal organs are also present, as well as a reddish colouration of some areas of the rock which is taken as presence of haemoglobin in the blood of Platecarpus. Haemoglobin is the part of red blood cells that carry oxygen, as formed by the intake of iron as part of the diet (as a predator Platecarpus would have gotten this from digesting prey that had in turn digested trace amounts of iron in their diets). Just as iron can be seen to rust into a brownish red colour, the iron from the haemoglobin can stain rocks into a similar colour.
Another key area that reveals a valuable insight into mosasaur biology is the preservation of cartilaginous rings of the trachea. Commonly known as the windpipe this carried air to the lungs, but the key revelation here is that the junction of the bronchi is also preserved. The fact that this actually splits into two is evidence that mosasaurs had two working lungs. This allowed for a greater inference for mosasaur evolution as one theory was that the mosasaurs evolved from terrestrial monitor lizards while another suggested that their ancestors were snakes. Snakes though usually have one lung that is considerably more developed than another, the weaker lung being barely functional to vestigial (present but no longer used); while in other snakes the lung is completely absent. The split in the trachea suggests two working lungs and a monitor lizard lineage (Later discoveries such as Dallasaurus and Russellosaurus further support this theory). The area where the junction occurs is also interesting as it takes place before the trachea passes beneath the forelimbs, whereas in land lizards the junction is at the same point as the forelimbs. The area of the digestive tract shows a concentration of fish parts such as scales, not only revealing the prey specialisation of Platecarpus, but giving a clue to its metabolism and internal workings. The fact that material passes through so quickly that harder parts like scales are not fully processes suggests that the digestive system was incapable of doing so. This could suggest a system that was close to operating upon the principals of a cold blooded metabolism. Cold blooded metabolisms take longer to process food and as such increases the risk of it decomposing inside the body before it’s digested, poisoning the animal in the process. This is why many reptiles will not eat if they are too cold, and may even regurgitate food rather than having it rot inside them. In the case of Platecarpus (and also for another mosasaur named Globidens) it would seem that prey was only digested for the softer fleshier parts while the harder parts were passed out as waste rather than being left in the system.
It would however be an error to assume mosasaurs were just sluggish marine lizards. The Cretaceous seas were warmer than what they are today, and there are other factors to consider as well like potential gigantothermy. In short this is where an animal has a low surface area to body mass ratio (i.e. thickly built) which means the very body itself can insulate the internal parts so that they retain heat. This increases the internal metabolism, and evidence for this occurrence can be seen today in so called ‘traditionally cold-blooded’ animals like large sharks. These factors combined with the currently available fossil evidence can be taken as suggesting that mosasaurs had cold-blooded metabolisms, but were possibly slightly higher than their related terrestrial ancestors.
Skin impressions show that Platecarpus had different scales over different parts of its body. On the main body and under the tail the scales were rhomboid and arranged in diagonal rows, but most importantly they were arranged so that the back of the scales would overlap the scales of the row behind them. This arrangement suggests that the scales were developed to increase hydro-dynamic efficiency by reducing resistance and allowing water to pass over them as Platecarpus swam forwards. The scales on the upper portions of the tail however were similar to those on the body but larger, possibly to help with the rigidity of the tail (more on that in the section below). The scales on the tip and upper surface of the snout were hexagonal and did not touch one another, with these scales possibly occurring here because this was the part of the body that would have had the most contact with prey items. The scales on the jaws were similar to the body but longer, again possibly to increase hydro-dynamic efficiency.
Not much is known about Platecarpus’s olfactory ability (sense of smell), but a clue as to how well it was developed, as well as potential hunting behaviour comes from the fact that the nostrils are forward on the snout and face out sideways, something that may indicate a directional sense of smell. This is easy enough to explain when you think about how you have ears on the sides of your head. Unless a sound comes from directly in front or directly behind you, one ear nearest will always pick up the sound a fraction of a second before the other one. This may not sound much but that is all it takes for your brain to register the difference, which is why you might turn to face the direction of the sound even though you don’t actually make a conscious decision to do so. Nostrils facing out to the sides in opposite directions will both pick up scents, but the one closest to the direction of the source will register first. Also by being near the tip of the snout the nostrils would have been in the best possible place to pick up smells, and if Platecarpus detected blood it would not only know that they was either a fresh kill or wounded animal in the water, but which direction it was in. On a related note, mosasaurs like Platecarpus may have actually breathed in through their mouths when taking air on the surface as this would allow for a faster transference of air, which reduced their time on the surface where they may have been vulnerable to attack from other predators that they would not have been able to see coming up from below.
Not to forget the actual bones, the skull of Platecarpus is markedly different to other mosasaurs in that the skull and snout are proportionately shorter. It is feasible that this shortness may have been a visual aid as the orbits (the part of the skull where the eyes fit into) are angled to face slightly forwards, suggesting a degree of stereoscopic vision. Other mosasaurs, such as Mosasaurus itself are thought to have had lacked stereoscopic vision because their eyes faced more out to the sides with a large snout out in front that would have created a ‘blind spot’ right in front of the skull. The shorter snout of Platecarpus however meant that it did not have such a handicap, and it may have been able to gauge distances between itself and prey. This would give Platecarpus an edge in hunting smaller prey like fish which would require a good visual fix for effective capture. Platecarpus also has one of the lowest numbers of teeth among mosasaurs, in part a result of the shorter snout. The teeth themselves are not specifically adapted to work on just fish however, and as such Platecarpus may have occasionally tried for other prey types such as squid and ammonites.Platecarpus and the evidence for a mosasaur tail fluke
Platecarpus was long suspected as having a tail fluke similar to the primitive ichthyosaurs, something which was confirmed in a later discovery when a new Platecarpus specimen was discovered with soft tissue impressions that revealed it had a crescent shaped tail fluke. This discovery alone turned the study of mosasaurs upside down on its head as the long held reconstruction of mosasaurs gave them all long eel like tails that trailed behind their bodies.
The first clues for mosasaurs like Platecarpus having tail flukes came through focused study of one portion of the tail where the caudal vertebrae were very different in shape and proportion to the other vertebra. These vertebra had centra (the round central portion) that were shorter than the centra of other vertebrae which meant that these vertebrae could bend further. The neural spines of these vertebra however were wedge shaped, and just like the chevron shaped stones that you see in an archway these caused a naturally occurring bend in the tail when placed together which saw the tip pointed down at an angle. Impressions of soft tissue completed the picture with the presence of a crescent shaped tail fluke, although the lower lobe of the tail, the lobe the vertebrae and main body of tissue ran into was probably more developed. This tail is considered a case of convergent evolution as the same arrangement was seen in the early Triassic with the development of primitive ichthyosaurs.
It is not just the shape of the tail that is important however but the way that it worked. The neural spines of the vertebrae also have grooves which are thought to have been support for ligaments that ran down the vertebrae to stiffen the part of the tail that arced downwards. With the lower tail, which supported the fluke, stiffened, Platecarpus could make more efficient use of the fluke. It’s like if you think about paddling a canoe, your arms flex and move to drive the oar, but the oar is stiff so that more of the motion from your arms is transmitted into the water for increased push. As such Platecarpus would not have to expend energy in flexing muscles all the way down the tail, just to the point of the bend.
The fact that Platecarpus was developing a stronger swimming tail suggests that this was a mosasaur that was developing an increasing reliance upon speed in its hunting behaviour. As noted above the remains of fish have been found in concentrated deposits within Platecarpus fossils. Clearly this represents a shift towards fish as a primary prey source which makes Platecarpus different from the other mosasaurs that had a large prey preference, and as such it’s possible that the mosasaurs that went after larger and slower prey may have retained eel like tails, only needing to use a sudden burst of speed for ambushing tactics.
The newly acquired speed from developing this tail fluke would have seen mosasaurs like Platecarpus becoming the fastest known marine reptiles of the late Cretaceous oceans (advanced ichthyosaurs were probably faster but disappear from the fossil record at the end of the Turonian period ninety million years ago, long before Platecarpus appeared), and while larger mosasaurs were a potential threat, they may not have had the speed to catch smaller mosasaurs in a straight race. This meant that the only predators that were a threat in both killing ability and speed were fish, specifically larger Cretaceous sharks like Cretoxyrhina. Although sharks in general can only maintain strong bursts of speed for short periods, there is strong fossil evidence of Cretoxyrhina feeding upon marine reptiles. Since this article was first uploaded, another genus of mosasaur named Proganthodon that was once thought to have had an eel-like tail, has since been discovered to also have had a tail fluke similar to Platecarpus.
Further Reading
– On the reptilian orders Pythonomorpha and Streptosauria. – Proceedings of the Boston Society of Natural History 12:251-266 – Edward Drinker Cope – 1869. – A Mounted Skeleton of Platecarpus. – The Journal of Geology vol 18 – S. W. Williston – 1910. – A new genus of mosasaurs from Mexico, and notes on the pelvic girdle of Platecarpus. – Denison University Bulletin, Journal of the Scientific Laboratories 29(10):383-400. – M. G. Mehl – 1930. – The vertebrate fauna of the Selma Formation of Alabama. Part IV. The turtles of the family Toxochelyidae. – Fieldiana: Geology Memoirs 3(4):145-277 – R. Zangerl – 1953. – The Skull of American Mosasaurs – D. A. Russel – 1964. – Notes on mosasaurs from Texas. – The Texas Journal of Science 21(1):69-80. – J. T. Thurmond – 1969. The vertebrate fauna of the Selma Formation of Alabamam: Part VII The Mosasaurs. – Fieldiana: Geology Memoirs 3(7):365-380. – D. A. Russell – 1970. – The mosasaur “Angolasaurus” bocagei (Reptilia: Mosasauridae) from the Turonian of Angola re-interpreted as the earliest member of the genus Platecarpus. – Palaeont. Z. 68 (1/2): 267–282. – T. Lingham-Soliar – 1994. – Les mosasaures (Squamata) du Cretace Superieur du Bassin Basco-Cantabrique. – Geobios 20:19-26. – N. Bardet, J. C. Corral & X. Pereda Superbiola – 1997. – The marine vertebrate faunas from the Late Cretaceous phosphates of Syria. – Geological Magazine 137(3):269-290. – N. Bardet, H. Cappetta, X. Pereda Suberbiola, M. Mouty, A. K. Al Maleh, A. M. Ahmed, O. Khrata & N. Gannoum – 2000. – Stratigraphic distribution and habitat segregation of mosasaurs in the Upper Cretaceous of western and central Alabama, with an historical review of Alabama mosasaur discoveries. – Journal of Vertebrate Paleontology 22 (1): 91–103. – C. R. Kiernan – 2002 – First record of the mosasaur Platecarpus Cope, 1869 from South America and its systematic implications. – Revista Brasileira de Paleontologia 8(1):5-12. – P. Bengtson & J. Lindgren – 2005. – A review of Australian mosasaur occurences. Netherlands Journal of Geosciences – Geologie en Mijnbouw 84(3):307-313 – B. P. Kear, J. A. Long & J. E. Martin – 2005. – Tylosaurus kansasensis, a new species of tylosaurine (Squamata, Mosasauridae) from the Niobrara Chalk of western Kansas, USA. Netherlands Journal of Geosciences – Geologie en Mijnbouw 84(3):231-240 – M. J. Everhart – 2005. – Redescription of the holotype of Platecarpus tympaniticus Cope, 1869 (Mosasauridae: Plioplatecarpinae), and its implications for the alpha taxonomy of the genus. – Journal of Vertebrate Paleontology 30(5):1410-1421- T. Konishi, M. W. Caldwell & G. L. Bell Jr. – 2010. – Convergent evolution in aquatic tetrapods: insights from an exceptional fossil mosasaur. PLoS One 5(8):e11998 – J. Lindgren, M. W. Caldwell, T. Konishi & L. M. Chiappe – 2010. – Two new plioplatecarpine (Squamata, Mosasauridae) genera from the Upper Cretaceous of North America, and a global phylogenetic analysis of plioplatecarpines – Journal of Vertebrate Paleontology Vol 31, Issue 4 – Takuya Konishi & Michael W. Caldwell – 2011.